JuanJoseRuiz
Madmaxista
- Desde
- 2 Jun 2015
- Mensajes
- 21.707
- Reputación
- 42.518
EL bichito FUE CREADO Y SOLTADO A PROPOSITO PARA UN MACRO EXPERIMENTO DE NUEVA TECNOLOGIA DE banderillaS , INCLUSO PARA ALGUNOS CANCERES Y LA DEL SIDA
OBSERVESE ESTE PAPER DE NATURE DE 2018 YA VENIA TODO
 www.nature.com
www.nature.com
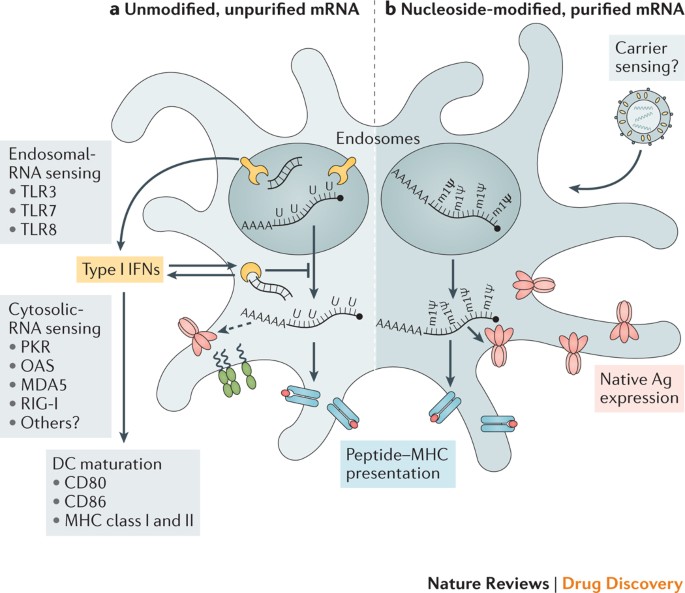
Potential safety concerns that are likely to be evaluated in future preclinical and clinical studies include local and systemic inflammation, the biodistribution and persistence of expressed immunogen, stimulation of auto-reactive antibodies and potential toxic effects of any non-native nucleotides and delivery system components. A possible concern could be that some mRNA-based vaccine platforms54,166 induce potent type I interferon responses, which have been associated not only with inflammation but also potentially with autoimmunity167,168. Thus, identification of individuals at an increased risk of autoimmune reactions before mRNA vaccination may allow reasonable precautions to be taken. Another potential safety issue could derive from the presence of extracellular RNA during mRNA vaccination. Extracellular naked RNA has been shown to increase the permeability of tightly packed endothelial cells and may thus contribute to oedema169. Another study showed that extracellular RNA promoted blood coagulation and pathological thrombus formation170. Safety will therefore need continued evaluation as different mRNA modalities and delivery systems are utilized for the first time in humans and are tested in larger patient populations.
Moderna Therapeutics, founded in 2010, has raised almost US$2 billion in capital with a plan to commercialize mRNA-based vaccines and therapies172,173. The US Biomedical Advanced Research and Development Authority (BARDA) has committed support for Moderna's clinical evaluation of a promising nucleoside-modified mRNA vaccine for Zika bichito (NCT03014089). In Germany, CureVac AG has an expanding portfolio of therapeutic targets174, including both cancer and infectious diseases, and BioNTech is developing an innovative approach to personalized cancer medicine using mRNA vaccines121 (Box 2). The tras*lation of basic research into clinical testing is also made more expedient by the commercialization of custom GMP products by companies such as New England Biolabs and Aldevron17
OBSERVESE ESTE PAPER DE NATURE DE 2018 YA VENIA TODO

mRNA vaccines — a new era in vaccinology - Nature Reviews Drug Discovery
mRNA vaccines represent a promising alternative to conventional vaccine approaches, but their application has been hampered by instability and delivery issues. Here, Pardi and colleagues discuss recent advances in mRNA vaccine technology, assess mRNA vaccines currently in development for cancer...
Potential safety concerns that are likely to be evaluated in future preclinical and clinical studies include local and systemic inflammation, the biodistribution and persistence of expressed immunogen, stimulation of auto-reactive antibodies and potential toxic effects of any non-native nucleotides and delivery system components. A possible concern could be that some mRNA-based vaccine platforms54,166 induce potent type I interferon responses, which have been associated not only with inflammation but also potentially with autoimmunity167,168. Thus, identification of individuals at an increased risk of autoimmune reactions before mRNA vaccination may allow reasonable precautions to be taken. Another potential safety issue could derive from the presence of extracellular RNA during mRNA vaccination. Extracellular naked RNA has been shown to increase the permeability of tightly packed endothelial cells and may thus contribute to oedema169. Another study showed that extracellular RNA promoted blood coagulation and pathological thrombus formation170. Safety will therefore need continued evaluation as different mRNA modalities and delivery systems are utilized for the first time in humans and are tested in larger patient populations.
Moderna Therapeutics, founded in 2010, has raised almost US$2 billion in capital with a plan to commercialize mRNA-based vaccines and therapies172,173. The US Biomedical Advanced Research and Development Authority (BARDA) has committed support for Moderna's clinical evaluation of a promising nucleoside-modified mRNA vaccine for Zika bichito (NCT03014089). In Germany, CureVac AG has an expanding portfolio of therapeutic targets174, including both cancer and infectious diseases, and BioNTech is developing an innovative approach to personalized cancer medicine using mRNA vaccines121 (Box 2). The tras*lation of basic research into clinical testing is also made more expedient by the commercialization of custom GMP products by companies such as New England Biolabs and Aldevron17
